Abstract
Background: PNH is characterized by a degree of bone marrow failure and hemolysis resulting in debilitating hemolytic anemia and an increased risk of thrombosis. Uncontrolled complement activation leads to intravascular hemolysis mediated by the membrane attack complex and extravascular hemolysis mediated by accumulation of C3 fragments, such as C3b, at the cell surface. The only approved treatment for PNH is Soliris®, a C5 inhibitor which targets intravascular hemolysis however, a significant number of patients treated with Soliris® continue to experience ongoing anemia and its associated symptoms. Due to the key role of C3 in the complement cascade, upstream of C5, APL-2, a cyclic peptide inhibitor of C3, acts to prevent both intravascular and extravascular hemolysis. To our knowledge these data represent the first time that PNH patients have experienced significant increases in haemoglobin (Hb) combined with normalisation of lactate dehydrogenase (LDH), absolute reticulocyte count (ARC) and total bilirubin.
Aims: This Phase Ib open-label, dose-escalation study being conducted in New Zealand, Thailand, Malaysia and Hong Kong was designed to assess the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and efficacy of multiple doses of APL-2, administered by daily subcutaneous injection (SC), in patients with PNH.
Methods: To be eligible for entry, PNH patients are required to have Hb levels < 105 g/L (normal range [NR] 119-180), LDH levels >2 times upper limit of normal (x ULN) at screening and have received ≥ 1 blood transfusion in the prior 12 months. The study will recruit 3 subjects into Cohort 1 and up to 20 subjects in Cohort 2 receiving 180 mg/d or 270 mg/d of APL-2, respectively. Efficacy is assessed by change from baseline in Hb, LDH, ARC, total bilirubin and transfusion requirements. Change from baseline in FACIT fatigue score is also reported.
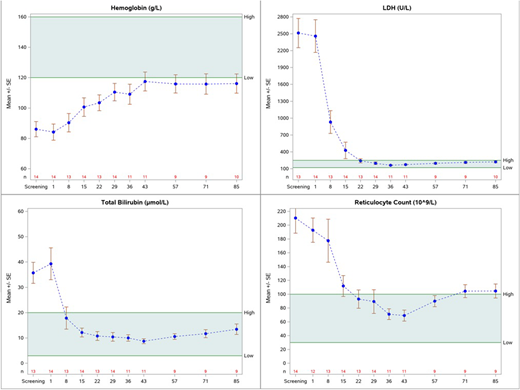
Results: As of 24 July 2018, Cohort 1 has been fully recruited and 17 subjects have been dosed in Cohort 2. Of the 17 subjects dosed in Cohort 2, 15 have been treated with APL-2 for >28 days and 9 have been treated for >84 days. Data is summarized for 14 (and 10) subjects in Cohort 2 who have received 270 mg/d of APL-2 for at least 28 (and 84) days; not included in the summary are 2 subjects who have not yet reached Day 28 and one subject who had underlying metastatic ovarian cancer with a chronic low gastrointestinal bleed, unknown at time of screening, resulting in artificially low Hb and high LDH levels determined to be unrelated to PNH. Baseline Hb was 84 g/L (range 55-110) which increased to 110 (74-135) and 116 (71-145) g/L at Days 29 and 85 respectively, representing a mean increase in Hb of 29 g/L and 33 g/L. At Day 29, Hb levels had increased in 100% of subjects with 57% achieving levels within the normal range. Hb increases were maintained in the 10 subjects with Day 85 data available. In the 12 months prior to APL-2 dosing, subjects received a total of 67 transfusions (average 4.8 per year per patient; range 0 to 15). Except for 2 subjects, each of whom received a single transfusion within the first two weeks of treatment i.e. before APL-2 had reached steady state concentration, no transfusions have been reported for any patient during the APL-2 treatment period. Mean baseline LDH of 2459 I/U (9.8x ULN) was reduced to 197 I/U (0.8x ULN) by Day 29. Mean baseline ARC and total bilirubin were reduced from 193 to 89 10^9/L (NR 30-100 10^9/L) and 39 umol/L to 10 umol/L (NR 3-15 umol/L), respectively. Of the 14 subjects, 13 (92%) had LDH and bilirubin within the normal range, and 11 (79%) had ARC in the normal range at Day 29. The Figure shows mean +/- SE for all available data for the 14 subjects through to Week 12. To date, APL-2 has been well-tolerated. No significant infections or thromboembolic events have been observed. One subject was withdrawn from the study due to progression of aplastic anemia related to underlying PNH. In clinical trials in PNH patients more than 5000 SC doses of APL-2 ≥ 270 mg/day have been administered, representing a cumulative systemic exposure of >700 patient weeks of APL-2 treatment.
Summary/Conclusions: We demonstrate that systemic inhibition of C3 with APL-2, controls both intravascular and extravascular hemolysis in patients with PNH as shown by significant reductions in LDH, total bilirubin and ARC. Broad control of hemolysis leads to significant and sustained increases in Hb in the absence of transfusions. APL-2 was safe and well tolerated.
Wong:Alexion: Honoraria. Deschatelets:Apellis Pharmaceuticals: Employment, Equity Ownership. Francois:Apellis Pharmaceuticals: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Hamdani:Apellis Pharmaceuticals: Employment, Equity Ownership. Johnson:Apellis Pharmaceuticals: Consultancy, Equity Ownership. Tan:Apellis Pharmacueticals: Consultancy. Tse:Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; MSD: Research Funding; Roche Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie Inc: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Grossi:Apellis Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract